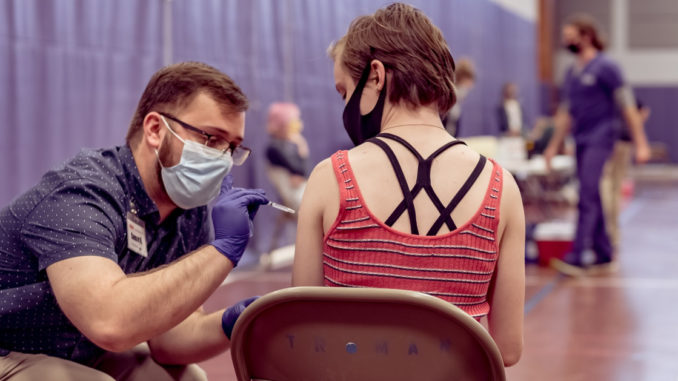
A COVID-19 vaccination clinic was held on campus for the Truman State University community April 7. Three hundred and sixty doses were administered at the event.
In a survey conducted by Nancy Daley-Moore and Scott Alberts, 644 students said they have already been vaccinated, and with the addition of the on-campus vaccination clinic, about one-fifth of Truman’s student population has been vaccinated.
Hy-Vee Pharmacy administered the Johnson & Johnson vaccine on campus with the help of nursing students and public health students. Brenda Higgins, associate vice president for student health and wellness, said the nursing students helped to draw up vaccines and to monitor patients for side effects. Public health students helped register students and helped with escorting them from station to station.
Junior Dylan Barclay got the vaccine at the on-campus clinic after looking for a way to get a vaccine for a while. He said the on-campus option was convenient as he could go right after his job.
“Overall, I’d say the experience was very good,” Barclay said. “While I had a lot of side effects from it and was sick for a few days, I’m still glad that I can be a bit safer, especially considering I’m close to a few people who are high-risk. It was pretty efficiently organized as well; everyone seemed to go through pretty quickly and I always had someone who was helping direct me.”
Barclay said social distancing was enforced, everyone was wearing masks and it wasn’t crowded at the event, all those factors together made him feel comfortable at the clinic.
Barclay said he was really glad the University had the pop-up clinic, as it made getting a vaccine quick, easy and convenient.
Higgins said Truman originally had 1,600 doses planned, but after opening up the first 400 and not filling all of those, they canceled the other planned event.
At this time, Higgins said she is not planning another vaccine clinic on Truman’s campus because it seems that all the people who wanted to be immunized were able to do so at the first clinic.
The Food and Drug Administration and the Centers for Disease Control and Prevention have now recommended a “pause” in the administration of the Johnson & Johnson COVID vaccine in order to allow investigation into a potential link between the vaccine and a rare blood clotting disorder in six recipients.
The on-campus clinic administered 300 doses of the Johnson & Johnson vaccine.
In an email to the Truman community April 13 Higgins wrote, the Emergency Use Authorization was not revoked, and the pause is being utilized as a precaution to allow further evaluation prior to proceeding with vaccinations.
Higgins also wrote that anyone who received this vaccine should be reassured that nationally only six individuals out of more than 6.8 million who received the vaccine have developed clotting disorders.
So, though serious, the adverse effects are extremely rare. Higgins wrote the COVID-19 virus is much more likely to cause clotting disorders than the Johnson & Johnson vaccine.
Higgins said the email was to advise anyone who received the Johnson & Johnson vaccine to watch for the symptoms of the rare clotting disorder and to notify your healthcare provider if you notice symptoms.
